Arnold S. E. Menke, 1965 · 1
Ammophila juncea, map |
Click on map for details about points.
|
|
Overview |
Taken from: A Revision of the North American Ammophila (hymenoptera, Sphecidae). Arnold S. E. Menke, 1965.
Ammophila (Ammophila) juncea Cresson
(figs. 5, 149)
Ammophila juncea Cresson, 1865. Proc. Entomol. Soc. Philadelphia 4:460. Holotype male, Colorado (Academy of Natural Sciences, Philadelphia)
Ammophila montezuma Cameron, 1888. Biologia Centrali-Americana, Hymen. 2:13. Holotype male, northern Sonora, Mexico (British Museum, London). New synonymy.
Male. Average length 21 mm., range: 15-25 mm.
Color. Black; petiole tergite red laterally, black above (western United States and some Floridian specimens) or entirely black (eastern United States); gastral segments I-II and sternite III, red, tergite I with elongate median black spot (western United States), gastral segments I-IV and most of V red, tergite I with black spot (California), or only gastral segment I red with dorsal black stripe (eastern United States), gastral segment I sometimes without dorsal black stripe (Florida); wings clear or slightly infumate (Florida), veins brown to black.
Vestiture. Gena with dense appressed silver hair (sometimes sparse or lacking in eastern specimens); collar and scutum with silver or brownish micropubescence (often absent in Floridian specimens); scutum with posteromedian longitudinal band of appressed silver hair; mesopleuron with band of appressed silver hair from base of mid coxa to top of hypoepimeral area; mesopleuron sparsely covered with appressed silver hair anteriorly (western United States); inferior metapleural area near hind coxal base and adjacent to metapleural sulcus with a band of appressed silver hair, band extending along sulcus nearly to superior metapleural pits, hairs of band generally parallel to plane of sulcus, band crossing sulcus in heavily pubescent specimens, sometimes reaching nearly to metapleural flange (fig. 54), propodeal hairs usually diagonal to sulcus, rarely perpendicular; inferior metapleural area usually weakly covered with appressed silver hair anteriorly (western United States); erect mesosomal hair extremely short in Floridian specimens.
Structure. Labrum truncate or slightly rounded; collar densely micropunctate, sparsely, shallowly and finely macropunctate scutum densely micropunctate, moderately shallowly macropunctate to densely macropunctate, or scutum coarsely striatopunctate (Florida); scutellum sparsely to moderately punctate anteriorly, feebly ridged posteriorly, or rugosopunctate (Florida); mesopleuron, inferior metapleural area, and propodeal side weakly micropunctate or etched, moderately macropunctate, or pleura coarsely striatopunctate (Florida); right penis valve similar to figures 128—129.
Female. Average length 21.5 mm., range: 16.5-23.5 mm.
Color. As in male except petiole tergite usually completely red, rarely black (South Carolina, New Jersey); gaster completely red (California, Nevada), or tergites III-V largely black (Arizona, Utah to Nebraska), or only segment I orange (eastern united States).
Vestiture. As in male except pleural bands more distinct; femoral psammophore hairs often brownish.
Structure. Labrum rounded but with a median projection; clypeal disk slightly bulging, densely micropunctate, moderately macropunctate, median free margin projecting, teeth blunt, rounded; inner orbits slightly to moderately converging below.
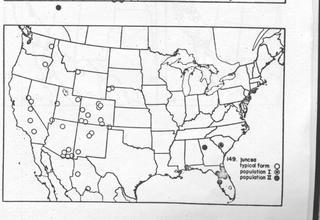
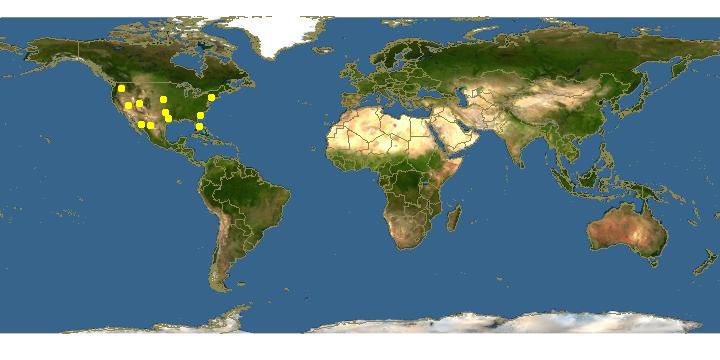
Range (fig. 149). Ammophila juncea has a peculiar disjunct distribution. The typical form occurs from the western great plains to California, Oren, and Washington and southward to northern Mexico. An eastern, darker colored form of juncea occurs along the Atlantic Coast from New York to Florida and Alabama. This eastern form is separated by a large gap from the typical western form of the species. Whether or not this split distribution is natural or the result of insufficient collecting remains to be seen. As discussed in the following paragraph on variation the eastern form of can be divided into two populations. Population I (New York, New Jersey, and South Carolina) is represented by circles with black centers on the distribution map. Population II (Alabama, Florida, and Georgia) is shown as solid circles. The typical western form of juncea is represented by open circles. The material studied in populations I and II is listed below.
Population I (7 males, 9 females, June to September): NEW JERSEY, Burlington Co.: Medford Lakes (CUI). Ocean Co.: Lakehurst (AMNH); Wranglebrook, Lakehurst (CUI). NEW YORK, Suffolk Co.: Yaphank, Long Island (AMNH). SOUTH CAROLINA, Aiken Co.: Aiken (CNC).
Population II (10 males, 6 females, May to October): ALABAMA, Mobile Co.: Mobile (CAS). FLORIDA, Clay Co.: Gold Head Branch State Park (USNM). Hillsborough Co.: Tampa (USNM). Levy Co.: “Levy Co.” (USNM). Marion Co.: Dunnellon (USNM); Orlando (LACM). Putnam Co.: Welaka (CUI). GEORGIA, Fulton Co.: Atlanta (UCD).
Variation. The only significant variation in western juncea is color and this has been adequately covered in the description. Population I of the eastern form is very similar to western juncea except for less red on the abdomen and sparser appressed genal hair. Population II differs from both population I and typical juncea in having a striatopunctate mesosoma. In addition the wings are lightly infumate and the erect mesosomal hair is very short and sparse in population II. The genal appressed hair is sparse in population II also. Because of the lack of material from the states between populations I and II it is not known whether II is a distinct entity or one that grades into I. However, since other species (urnaria for example) undergo similar, although admittedly less pronounced, changes in sculpture in the southeastern United States, I have decided to consider population II as conspecific with juncea. When the Ammophila fauna of the eastern United States is better known, especially in the southeast, it nay be necessary to reevaluate population II.
Systematics. The posteromedian silver scutal stripe generally offers a quick means of identifying juncea. A. hermosa has this stripe also but differs from juncea in having partially red legs.
Fernald (1931k) used the name juncea primarily for the species now known as cleopatra. Examination of Cresson’s type, however, has established the correct identity of juncea. Murray (1951) synonymized montezuma with aberti, but the type of montezuma is clearly synonymous with juncea. At least one of Fernald’s paratypes of floridensis is the same as population II of juncea.
Ammophila urnaria Group
Diagnosis. Primary characters: Free margin of male clypeus emarginate (except in aberti and bellula) hypostoma in male simple, without a process (fig. 29); collar and scutum usually without strong transverse ridges (except in cleopatra) propodeal enclosure irregularly rugose medially, diagonally ridged laterally, interspaces smooth and/or punctate, subshining to shining, or sometimes minutely etched, dull (fig. 22); preepisternal sulcus short, ending about opposite pronotal lobe; metapleural flange not lamellate (except in leoparda, kennedyi sometimes, and aberti rarely); spine-like process of penis valve head basal, generally directed toward base of penis valve stalk (except in bellula) (rigs. 122-225, 127- 129); base of gonoforceps not dorsoventrally elongate. Secondary characters : Clypeus and frons with appressed silver hair (except kennedyi and urnaria females), male clypeus usually completely covered by appressed hair, obscuring underlying sculpture (except kennedyi, picipes and some urnaria), female clypeus glabrous anteromedially; pronotal lobe and propodeum adjacent to petiole socket covered with appressed silver hair (lobe of female urnaria with dense hair only posteriorly); erect body hair pale; psammophore pale (sometimes partially brownish in dysmica, juncea, kennedyi, picipes and urnaria) length of flagellomere I in male less than least interocular distance and greater than length of flagellomere II; middle tibia with two veil developed apical spurs (except in some aberti and dysmica) forewing with three submarginal cells.
Included North American species. Ammophila aberti Haldeman, bellula Menke, cleopatra Menke, dysmica Menke, hermosa Menke, juncea Cresson, kennedyi (Murray), leoparda (Fernald), mescalero Menke, parkeri Menke, picipes Cameron and urnaria Dahlbom. Other New World species belonging to the urnaria group but not dealt with in this paper are: A. dejecta Cameron (Mexico), gracilis Lepeletier, lampei Strand, rufipes Guérin-Méneville, and suavis Burmeister (all South American).
Discussion. The urnaria group is a difficult one because most of the species are very similar. Also, the male genitalia display specific differences only in a few species, aberti, bellula and kennedyi. The penis valve head of urnaria is quite variable (figs. 128-129) and the following species have aedeagi of the same general configuration: cleopatra, dysmica, hermosa, juncea, leoparda, mescalero, parkeri and picipes. Of prime importance for species discrimination in the urnaria group ii the pattern of appressed hair on the mesosoma, particularly the pleural region. Unfortunately, old or worn specimens make hair patterns difficult to use, and often it is impossible to identify material in this condition without a thorough knowledge of the group. Some species have characteristic markings on the abdomen and a few have bicolored legs or other chromatic peculiarities. Structurally the urnaria group is fairly homogeneous with nearly all species having similar punctation etc. Some differences are found in the shape of the labrum but here also there is variability. Clypeal structure is sometimes distinctive but it is difficult to describe. The possibility of using various head measurements in species diagnosis has not been fully explored in the urnaria group. Comparisons of the lengths of flagellomeres I and II and also of the least interocular distance versus the length of flagellomere I seem to have no value, at least for the distinction of difficult species. Other measurements might be made in search of better species criteria: overall head width, eye width, length of head and degree of eye convergence, for example.
The six species that are the most difficult to identity can be divided into two groups of black-legged species. In one group, the mesopleural band of appressed hair ends at the bottom of the hypoepimeral area (dysmica, kennedyi and urnaria). In the second group the band extends to the top of the hypoepimeral area juncea, and picipes). A few species in the urnaria group seem to have no close allies: Ammophila aberti, bellula, leoparda and mescalero.
The arvensis group proposed by Fernald (1934) contained species belonging to the urnaria group and the azteca group. Fernald’s concept included pilosa (Fernald) (= azteca Cameron), arvensis Dahlbom (an Old World name used in error for mediata Cresson, evansi Menke and possibly other species), floridensis (Fernald) (= urnaria Dahlbom), urnaria Dahlbom (Fernald confused kennedyi and possibly other species with urnaria), and leoparda (Fernald) as variety of urnaria). The similar pleural pubescence pattern exhibited by these species was apparently the only criterion used by Fernald for this unnatural assumblage of species.
The urnaria group is represented throughout the New World, but appears to have no Old World allies.
|
|
|
Names | |
|
|
| Supported by | |
Updated: 2024-04-25 19:32:28 gmt
|